Three 'Organs-on-Chips'ready to serve as disease models, drug testbeds
(BOSTON) — Three organs-on-a-chip devices mimic human organ functions in a format amenable for drug testing, Wyss Institute researchers now report. The results bring Organs-on-Chips a big step closer to being accurate alternatives to animal tests for new drugs and toxins.
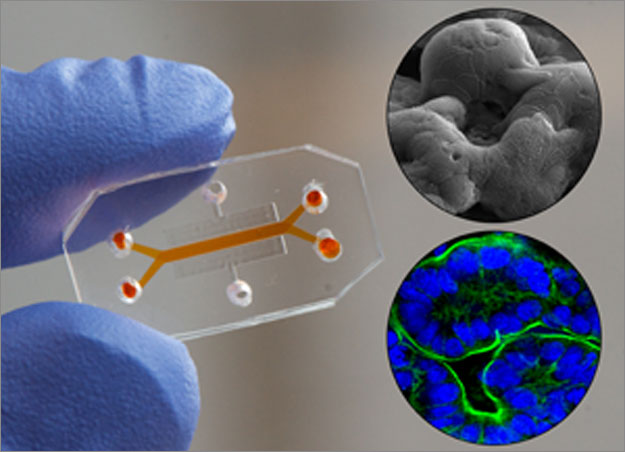
The results appeared in three articles in a special joint issue of the journals Integrative Biology and Lab-on-a-Chip, which joined forces to highlight this growing field. Wyss Institute Founding Director Don Ingber, M.D., Ph.D., who leads the Organs-on-Chips effort at the Institute, co-edited the special issue.
"Mice are not humans, and tests on animals often fail to mimic human diseases or predict how the human body responds to new drugs," Ingber said. "Organs-on-Chips like these three could replace many animal tests, which would fundamentally alter medical research, drug discovery and development, and safety testing of chemicals." Ingber, who is the senior author of separate articles in the issue on kidney- and gut-on-a-chip devices, is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at Harvard School of Engineering and Applied Sciences (SEAS).
A third article in the issue described development of a heart-on-a-chip microdevice that offers an improved way to assess cardiac toxicity. The device was developed by a team led by Kevin Kit Parker, who is a Wyss Institute Founding Core Faculty member and the Tarr Family Professor of Bioengineering and Applied Physics at SEAS.
Organs-on-chips are small microfluidic devices with hollow channels lined by living human cells. They have several properties that make them more realistic models of human organs than conventional lab-grown cells. Ordinarily, lab-grown cells sit in a bath of nutrient liquid in a plastic dish. But in organs-on-chips, human cells are cultured within hollow channels through which nutrient liquid flows. This allows researchers to alter the mix of chemical compounds that the cells encounter, as well as fluid shear forces that can influence cell behavior.
Moreover, the cells are arranged in the device to mimic key structural features of the internal architecture of the organ, and the devices can alternately stretch and relax to mimic distortions cells experience in our bodies. Such mechanical forces provide cues that are critical for cellular function — for example, the wavelike contractions of the gut wall during peristalsis turn out to be crucial for intestinal development and function.
Together, these properties of organs-on-chips allow scientists to analyze dynamic interactions between drugs and cells that can normally only be measured in living animals.
For their kidney-on-a-chip, Ingber, Kyung-Jin Jang, a postdoctoral fellow at the Wyss Institute, and their colleagues grew cells from a portion of a healthy human kidney called the proximal tubule on a porous membrane inside the device, and adjusted the fluid flow to what cells experience inside the living organ in our bodies.
Cells cultured this way developed structures normally seen only in living kidneys, including tight seals between cells, a ruffled cell surface, and hair-like projections called cilia. They behaved realistically, too. For example, the researchers tested a widely used cancer chemotherapy drug called cisplatin that is known to cause kidney toxicity in humans, but not in animals. They found that it injured the human cells in the kidney-on-a-chip, and the cells bounced back within days after the exposure ended — just as they do in cancer patients after doctors stop giving them cisplatin.
"This means this system could potentially be used as a drug-screening tool to study human kidney-cell responses that we can not observe in animal tests," Jang said.
A gut-on-a-chip developed by Ingber’s team enabled cultured human intestinal cells to regenerate into a specialized tissue that looked and functioned like the lining of the human small intestine. In the device, the cultured cells are stretched and released to mimic the forces they’d experience during peristalsis, and fluid trickles over the tissue as it does in the human gut.
"We want these cells to feel like they’re at home," said Hyun Jung Kim, the lead author of the paper and a Technology Development Fellow at the Wyss Institute.
While Ingber, Kim and their colleagues first reported the gut-on-a-chip last year, they now show that the device’s lifelike conditions cause cultured gut cells to morph into the four known types of gut-lining cells. The cells spontaneously arranged themselves into finger-like projections called villi that are normally seen only in living intestine. What’s more, the cells produce mucus and increase production of cytochrome P450 — an enzyme that breaks down drugs.
For these reasons, the gut-on-a-chip could provide more accurate predictions of drug absorption in the gut than any cell culture tests now available, which could indicate whether a new drug can be delivered as a pill. It could also serve as a powerful new tool for studying intestinal physiology and digestive diseases.
The heart-on-a-chip microdevice developed by Kevin Kit Parker and his colleagues offers an improved way to see if new drugs harm the heart. Heart toxicity is one of the leading causes for drugs withdrawal from the market. Today scientists measure cardiac toxicity by testing isolated, lab-grown heart cells to see if new drugs alter an enzyme critical to transmitting the electrical signals that control the heartbeat.
But in the heart-on-a-chip cells line up in parallel as they would in the wall of a real heart, and they coordinate their efforts to beat as one. The cells sit on a parallel array of thin, bendable plastic tabs, and the scientists gauge the strength of the heart beat by measuring how much the tabs bend when cells contract, said Ashutosh Agarwal, a Wyss Institute postdoctoral fellow who is the lead author of the new heart-on-a-chip paper.

Parker, Agarwal, and their colleagues created a microfluidic device that lets them observe the heartbeat through the device’s clear plastic window. They also developed a semi-automated, laser-based method to make the array of bendable tabs.
When the Wyss researchers bathed the heart tissue with a drug used to help strengthen a weakly contracting heart, the tissue responded as an intact heart would. This means that it could provide a realistic way to detect cardiac side effects without testing the drugs on animals.
"Since new and more realistic tests for cardiac toxicity are urgently needed, our heart-on-a-chip could catch on quickly, and we’re pushing hard to deliver a device that drug companies need," Parker said.
"We urgently need faster and better ways to develop new drugs," Ingber said. "Kit’s heart-on-a-chip and our gut- and kidney chip provide some of the most realistic models yet to study how drugs affect these organs in humans, and this could speed up drug development," Ingber said.
Ingber’s and Parker’s work was funded by the Wyss Institute for Biologically Inspired Engineering at Harvard University, the Defense Advanced Research Projects Agency (DARPA), National Institutes of Health, National Institute of Environmental Health Sciences, and National Science Foundation. In addition to Jang and Ingber, the kidney-on-a-chip research team included several other Wyss Institute staff, including: Ali Poyan Mehr, M.D., a clinical fellow; Geraldine Hamilton, Ph.D., a senior staff scientist; Lori McPartlin, Ph.D., a research associate; Seyoon Chung, Ph.D., and the late Kahp-Yang Suh, Ph.D., who was an Assistant Professor at Seoul National University in South Korea. In addition to Agarwal and Parker, the heart-on-a-chip research team included: Josue Adrian Goss, laboratory manager for Parker’s team; Alexander Cho, an undergraduate researcher, and Megan Laura McCain, Ph.D., a postdoctoral fellow.