Scientists have been trying for years to design the “Goldilocks” of biocompatible materials that are “just right” for tissue engineering applications — not too difficult to make, elastic enough to respond to the dynamic nature of the human body, and stable enough to support effective cell growth.
However, most materials designed so far fall short.
An international team led by the Wyss Institute recently used microfabrication techniques to design a new micropatterned hydrogel that shows great promise for tissue engineering — cardiac tissue in particular. It incorporates an elastic protein called tropoelastin, which is found in all elastic human tissues.
As reported recently in Biomaterials, these so-called “MeTro hydrogels” are highly elastic, and can be fabricated in less than one minute. Lead author Nasim Annabi, Ph.D., a Wyss Postdoctoral Fellow, said that the gel recipe calls for ultraviolet light, which is used to create a reactive form of tropoelastin called methacrylated tropoelastin in a water-based solution — hence the name “MeTro hydrogel.”
The MeTro gels extend up to 400 percent of their length before breaking. They also exhibit superior mechanical properties overall and support robust cell growth both in two-dimensional and three-dimensional culture environments, said Annabi, who collaborated with Wyss Associate Faculty member Ali Khademhosseini, Ph.D., others from the United States, and a team from the University of Sydney in Australia led by Anthony Weiss, Ph.D.
“It’s a nice stable gel,” said Annabi, “and it is tunable, too,” — meaning she can safely tweak the gel to change its physical properties, such as porosity, swelling properties, elasticity, and stiffness.
But there’s even more to get the heart pumping in this story.
In a tandem publication in another journal called Advanced Functional Materials, the team recently reported their success using the MeTro hydrogels to successfully engineer cardiac tissue.
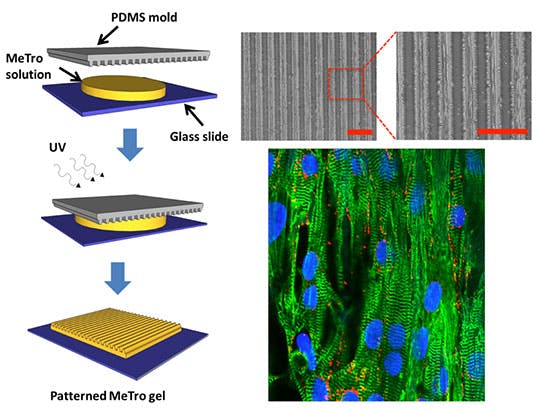
The researchers mimicked the nature of human heart tissue by adding linear patterns to the gels to align heart cells and engineer cardiac tissues. “To the best of our knowledge,” Annabi said, “this is the first report of purpose-built, high-resolution patterns on the surface of an elastic human protein-based gel.”
The gels promoted the attachment, spreading, alignment, function, and intercellular communication of heart cells isolated from the rat heart by providing an elastic mechanical support that mimics their dynamic properties in vivo, she said. They even contract in synchrony on these elastic substrates, in response to electrical stimulation.
The team’s next step is to see if the MeTro gels will effectively regenerate heart tissue in the artery of a sheep. Eventually, MeTro gels could be used to engineer new heart valves or in model cardiac diseases, Annabi said.
The team acknowledges research support by the National Health and Medical Research Council, the National Science Foundation, the Office of Naval Research, the National Institutes of Health, the CRC for Polymers, Australian Research Council, Australian Defense Health Foundation, and the National Health and Medical Research Council.