Scientists have been on the hunt for better ways to study cells that operate in a "code blue" condition called ischemia, which can occur during a heart attack or stroke. A novel, three-dimensional, paper-based system developed by Harvard scientists could help. The system mimics the tissue environment around a blocked coronary artery better than conventional cell culture systems, as reported recently in Advanced Healthcare Materials.
During ischemia, a blocked blood vessel deprives cells within the surrounding tissue of the oxygen and nutrients they need to carry out normal cellular metabolism, and conditions in the tissue change quickly as they become depleted.
Most of the traditional cell culture systems scientists use to study the disorder fail to mimic these conditions, and they are either difficult to analyze or do not allow scientists to study more than one cell type at a time. As such, they are akin to studying a fish in a bowl to elucidate its role in the sea; a lot of critical information is lost.
"There is a generally accepted need in the biology community for a simple yet sophisticated cell culture system that is physiologically relevant," said Wyss Institute Postdoctoral Fellow Bobak Mosadegh, Ph.D.
The new paper-based solution, which Mosadegh helped develop, lets the scientists culture more than one cell type at the same time, and observe how the cells interact with each other — a critical achievement because cells are constantly communicating with each other to impact tissue-level function.
The team, led by Wyss Institute Core Faculty member George Whitesides, Ph.D., who is also the Woodford L. and Ann A. Flowers University Professor at Harvard University, included Mosadegh, the lead author; Wyss Core Faculty member Kit Parker, Ph.D., who is also the Tarr Family Professor of Bioengineering and Applied Physics at SEAS; Borna Dabiri, a graduate student at SEAS, and others.
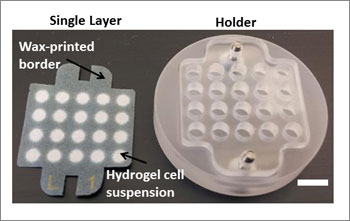
The advance builds on a 3D cell culture system that another Whitesides-led team introduced, in collaboration with Vertex Pharmaceuticals, in 2009 called " Cells in Gels in Paper." This system uses stacks of wax-printed paper as scaffolds that support hydrogels containing cells. Cells suspended in a hydrogel are introduced into the areas on the paper that do not have wax. Scientists assemble the papers into stacks that resemble whichever tissue constructs they are trying to mimic.
This modular system has outperformed other cell culture systems such as Petri dishes, where cells are grown in unnaturally thin monolayers, and hydrogel-only culture systems, which are difficult to analyze.
"The power of the paper-based system is that it is easy to assemble thick tissue-like constructs of defined composition, and even easier to disassemble the constructs back into thin layers for analysis," Mosadegh said.
The gravitas of the gradient
In healthy heart tissue, blood vessels supply oxygen, glucose and other nutrients to the cells surrounding the vessels while also removing the byproducts of cellular metabolism, such as carbon dioxide. A natural gradient forms around the tissues; the concentration of oxygen and nutrients is high close to the blood vessel, and decreases the further you move away from the vessel. The body tries hard to ensure that the distance between blood vessels is small enough so that cells are adequately supplied with nutrients. If a vessel becomes blocked, however, the overall rate of oxygen and nutrient transport decreases, and cellular byproducts accumulate.
Conventional cell culture systems only allow scientists to introduce a uniform level of reduced oxygen to the cell cultures to mimic the hypoxic conditions associated with ischemia. These fail to recapitulate the oxygen gradient that is so critical to whether ischemic tissues survive.
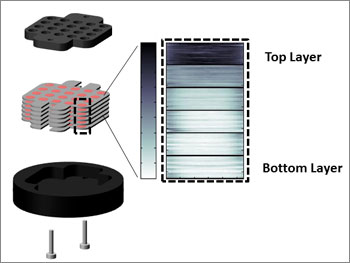
To mimic the natural, dynamic environment of a tissue surrounding a blocked coronary artery, the scientists first constructed a six-layer stack of papers about one millimeter thick. The layers contained heart cells from a rat. They laid the paper in a solid holder made of a cell-friendly, plastic material, and introduced oxygenated nutrient liquid from the top only.
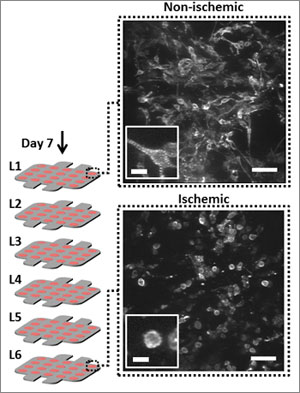
The cells at the top — closer to the source — remained healthy as they consumed the oxygen and nutrients, while those at the bottom resembled what you would see in an ischemic tissue — mimicking what happens in the human body. Gradient achieved.
Cell morphology changed as well: those at the top were elongated, just as you would expect for a healthy heart cell, while those at the bottom were more rounded in shape, a sign of less healthy cells.
Next they took a thick stack of papers, and a shorter stack of papers. Since oxygen and nutrients have further to diffuse through thicker stacks, the scientists expected cells at the bottom of thicker stacks to be under greater oxygen and nutrient stress than those at the bottom of shorter stacks. They tested this idea by introducing two kinds of cells to the stack: heart cells and fibroblasts, which are cells that play a critical role in wound healing. In ischemic heart tissue, fibroblasts respond to chemical distress calls from metabolically stressed heart cells, growing and moving against the oxygen gradient to help repair the tissue.
The researchers positioned the heart cells below those loaded with fibroblasts in the stack of papers. As expected, they observed more ischemia in the thicker stacks than in the shorter stacks.
They also demonstrated that the cells communicated with each other in these tissue-like constructs. A greater number of fibroblasts migrated towards the ischemic heart cells in the thicker stacks — moving away from the oxygen source to do so — than in the shorter stacks.
"This simple technology enables us to do very sophisticated co-cultures with relatively few resources," said Whitesides. "It presents a robust, scalable model for people to study drug delivery within tissue-like environments."
The team’s next step is to study how well this system matches the environment of tissues in the context of drug delivery, Mosadegh said. They will also continue to optimize how the cells behave in the tissue-like paper constructs. The best performing cell culture systems in terms of cell behavior are microfluidic devices such as the organs-on-chips, pioneered by Founding Director Don Ingber at the Wyss Institute.
"Perhaps the paper-based system can be used as a simpler screening tool before a drug candidate is tested on the organs-on-chips," Mosadegh said.
This work was funded by the Wyss Institute for Biologically Inspired Engineering at Harvard University, National Institutes of Health, Vertex Pharmaceuticals Incorporated, and MRSEC.