eGenesis works toward ending the global transplant shortage and transforming the treatment of organ failure.
The Problem
The demand for organ transplants significantly outweighs the available supply. According to the United States Health Resources and Services Administration, there are currently more than 100,000 people on the U.S. national transplant waiting list, and only about 46,000 transplant procedures were performed in 2023. With a new patient added to the waiting list about every eight minutes, this shortage leads to an average of 17 people dying each day while waiting for a donor organ.
According to the American Kidney Fund, kidney disease in particular affects more than 14% of US adults, with more than 800,000 living with kidney failure. Nearly 550,000 of these patients are treated with dialysis to clean their blood, and about 25,000 kidney transplants are performed each year.
Scientists have long investigated the use of animal organs as a potential solution to the organ shortage crisis but significant challenges stood in the way of these “xenotransplantation” approaches. One major issue is endogenous retroviruses present in animals’ DNA that can be transmitted to humans, which poses a risk of infection. Another critical problem is the human immune system’s tendency to reject animal organs, recognizing them as foreign and attacking them, which can lead to organ failure.
Our Solution
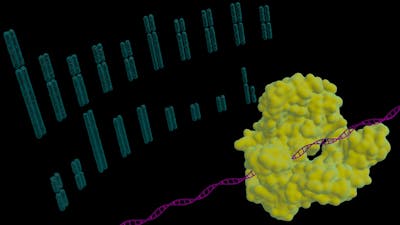
In 2015, Wyss Core Faculty member George Church, Ph.D. and a team spearheaded by his Postdoctoral Fellow Luhan Yang, Ph.D. at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard Medical School (HMS) used the CRISPR-Cas9 gene editing system to inactivate all 62 copies of porcine endogenous retrovirus (PERV) found natively in the genome of pigs. The significantly genome-edited kidney cells from pigs opened the door to pig-to-human xenotransplantation, and the novel approach was published in a landmark article in Science.
Product Journey

In 2016, eGenesis launched from the Wyss and HMS to build on the team’s success and develop safe and effective pig organs for transplantation into human patients. In the subsequent years, transplant clinicians at the Massachusetts General Hospital (MGH), scientists from eGenesis, and Church’s team continued to collaborate on this highly promising research.
Impact
In a groundbreaking procedure performed at MGH, Richard “Rick” Slayman, 62, became the first patient ever to receive a life-saving organ transplant from a pig donor. On March 16, 2024, talented surgeons successfully connected a CRISPR-modified pig kidney provided by eGenesis to Slayman, who had end-stage renal failure and was not a candidate anymore for a transplant from a human donor. The modified kidney, besides the inactivation of endogenous retroviruses, contained additional genome modifications to ensure it was safe for and compatible with human patients. Within a month, Slayman had recovered vital kidney functions and was discharged from the hospital to return home.
While recovering at home, Slayman passed away from an “unexpected cardiac event. Despite Slayman’s passing, his successful transplant is already being hailed as a milestone in xenotransplantation research and a beacon of hope to countless transplant patients worldwide.
Slayman’s procedure was performed under the FDA Expanded Access, or “compassionate use” protocol, which is granted for patients who have a serious or immediately life-threatening disease or condition, enabling them access to not-yet-approved treatment outside of clinical trials when no comparable therapy options are available. In September 2024, eGenesis announced they raised $191M to advance toward its first clinical trials.
On January 25, 2025, surgeons within Mass General Transplant Center performed the successful completion of its second transplant of an eGenesis-supplied genetically edited pig kidney into a living recipient. The patient, Tim Andrews, had been on dialysis for more than two years due to end-stage kidney disease before receiving the kidney.
“As soon as I woke up after the surgery, the cloud of dialysis disappeared. I felt re-energized and revitalized. It was a miracle,” Andrews said. “There are more than 500,000 people on dialysis, and I want to inspire them to never give up hope because that’s what this transplant provides. It’s a glimmer of hope.”
In February 2025, the FDA went beyond compassionate use cases for these transplants and authorized eGenesis and another company to begin clinical trials with modified pig kidneys into people with kidney failure, paving the way, if successful, for broader regulatory approval for the procedure.
In addition to kidneys, eGenesis is also currently developing liver and heart solutions to address the urgent needs of patients with other organ failures.