Biofilms are the bacterial slippery deposits found on many synthetic and biological surfaces and have been wreaking havoc on many areas of industrial productivity and human health. However, scientists have built sufficient understanding of them to begin to subvert their properties from within to help rather then harm.
Building on previous work, a team at the Wyss Institute for Biologically Inspired Engineering led by Core Faculty member Neel Joshi has added a new function to genetically engineered biofilms: the ability to incorporate enzymes. Enzymes are the molecular machines that perform the bulk of chemical reactions in all organisms, which means enzyme-coupled biofilms could become a new tool for drug makers, as well as important players in the cleanup of wastewater and contaminated soil.
BIND variations
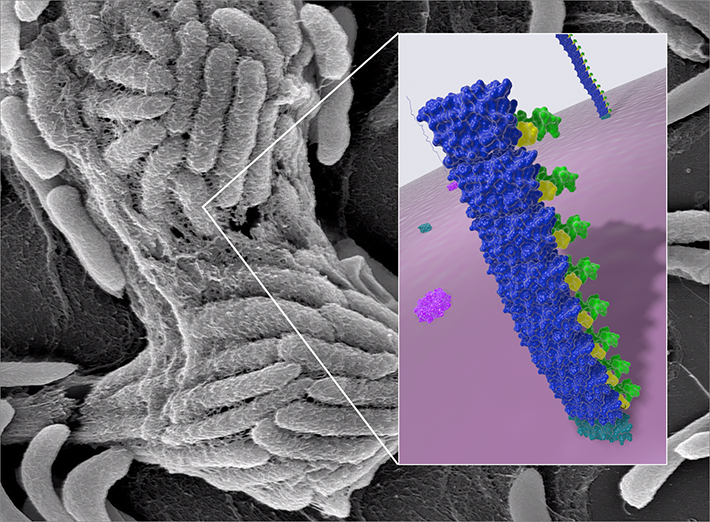
Last year, the Wyss team published their first comprehensive foray into the world of genetically engineered biofilms. The team developed a technology called Biofilm-Integrated Nanofiber Display (BIND), which leverages the biofilm-generating molecular machinery in E. coli bacteria. E. coli secrete a group of proteins from the inside of bacterial cells into their immediate environment, where they self-assemble into a tight network of polymeric nanofibers, creating the biofilm.
The team genetically endowed the key extracellular protein, CsgA, with novel functions that enabled biofilms to attach to industrial surfaces, synthesize miniscule particles as vehicles for drug delivery or capture and stably present foreign proteins in its environment.
In a second study, published in Biotechnology and Bioengineering, the Wyss scientists now further varied the BIND theme by recruiting a first model enzyme, the starch-digesting amylase into a genetically engineered biofilm. Enzymes generally convert specific substrates into differently structured products in a chemical reaction that is generally known as biocatalysis. To achieve the first biocatalytic BIND, the team used a modular conjugation system to fuse the biofilm protein CsgA to a small peptide, the Spytag. The amylase enzyme as the second component was fused to a larger protein called a Spycatcher, which recognized the Spytag and thereby recruited amylase into the biofilm.
Biocatalysis in the biotechnological and pharmaceutical industry can provide clear advantages over conventional chemical reactions because dedicated enzymes can modify their substrates in very complex ways and with extraordinary specificity.
"Due to its modular architecture with the broadly applicable Spycatcher system, this BIND strategy can be applied to almost any type of enzyme," said Zsofia Botyanszki, a graduate student of Joshi and the study’s first author. "This makes it a highly versatile platform for enzyme immobilization and biocatalysis of a great number of valuable reactions."
Working with enzymes is challenging because, their stability deteriorates once they are exposed to the harsh chemical working environment that certain reactions require. However, when the amylase enzyme was introduced into biofilms, the team found that its activity was stable over a range of chemical conditions and hardly declined within the four weeks following BIND construction. The biofilm environment protected the embedded enzyme molecules, therefore boosting their half-lives and making the reaction more robust.
Towards translation
The Wyss team is currently working to translate biocatalytic BINDs for a variety of applications in biotechnology: "The most likely first real-life applications will be in the synthesis of fine chemicals, like pharmaceuticals,” said Joshi, who is an Associate Professor of Chemical and Biological Engineering at the Harvard School of Engineering and Applied Sciences (SEAS). “Most pharmaceutical companies already use enzymes extensively in their synthetic workflows and many are looking for systems that can enhance or preserve the activity of those enzymes. Also, the regulatory hurdles are lower in a controlled system."
The Wyss team is now testing a panel of enzymes and enzyme combinations and is fine-tuning the ability of BINDS to adhere to reaction-compatible industrial surfaces. On a second venue, the Wyss group is going to investigate waste-water treatment and environmental cleanup applications of biocatalytic BINDs.