Harvard's Wyss Institute and Children's Hospital Boston Collaborate on Research Initiative
A research collaboration between Children’s Hospital Boston and the Wyss Institute for Biologically Inspired Engineering at Harvard University has identified a novel role for mechanical forces in control of whole organ formation. The findings, which appear in the current issue of Developmental Cell, could lead to a new approach for organ engineering in humans.
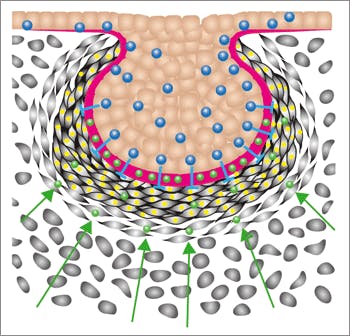
Scientists have long recognized that genes and molecules play a critical role in initiating organ formation; however, little is known about how these cues guide cells to join together to form a three-dimensional organ composed of multiple interacting tissues, such as a lung, kidney or even a tooth.
Wyss Institute Founding Director Donald Ingber, MD, PhD, and Tadanori Mammoto, MD, PhD, a Research Fellow in the Vascular Biology Program at Children’s Hospital Boston, led an interdisciplinary research team of engineers, geneticists, biochemists, and cell biologists to unlock some of these secrets. Ingber is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Children’s Hospital Boston, and Professor of Bioengineering at Harvard’s School of Engineering and Applied Sciences.
Focusing on the tooth as one of the simplest models of organ formation, their team found that the genes that encode biochemical factors that stimulate cell migration also trigger tooth formation by causing connective tissue cells to migrate towards each other and form a compact cell mass, or what is known as a “condensed mesenchyme.” For over a century, this process has been known to be critical for initiation of multiple types of organs, ranging from tooth, pancreas, and mammary gland to cartilage, but the underlying mechanism has remained a mystery.
The combined Wyss Institute – Children’s Hospital Boston team discovered that the physical process of compressing and rounding these cells is sufficient to trigger expression of the transcription factors that drive subsequent tooth development. For example, the researchers were able to induce formation of a whole tooth covered by enamel and dentin by mechanically squeezing the connective tissue cells between two rubber plates and then implanting the tissue in an animal.
The long-term goal of the team is to engineer synthetic scaffolds that, when implanted in the oral cavity, will produce the same mechanical transformations necessary to induce whole tooth formation in humans. Proving the viability of the concept in this simple organ model will be a promising first step toward being able to induce formation of more complex organ tissues, such as a liver, kidney, or liver.
“By bringing together these complementary institutions and synergistic areas of expertise, we were able to shed new light on the fundamental principles governing organ formation,” said Ingber. “We hope to apply these lessons to develop new bioinspired inductive materials that will ultimately help the millions of people who suffer from organ failure–and help transform medicine in the process.”
The collaboration is part of SysCODE (Systems-Based Consortium for Organ Design and Engineering), a group of seven clinical and academic institutions working to develop new ways to induce organ regeneration.