Method could advance biology and spur new approaches to disease
By Mark Dwortzan, Boston University College of Engineering
BOSTON — Cell traction forces, the tugging of individual cells on their immediate environment, play an important role in fundamental biological processes such as cell division, differentiation and migration, and more complex ones such as embryonic development and inflammation. When receptors on the cell surface called integrins adhere to the cell’s surrounding network of protein scaffolding known as the extracellular matrix, the forces they exert signal to the rest of the cell that important cellular processes are underway.
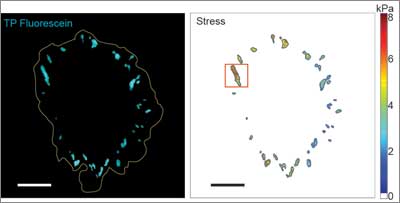
Measuring those forces over time could reveal more about the inner workings of normal and abnormal cellular development, and enable clinicians to more effectively diagnose and treat cancer, scarring and inflammation. Overcoming several limitations of conventional approaches, a new method developed by Professor Christopher Chen and collaborators at several institutions—including Harvard University, Stanford University, University of Pennsylvania, the Wyss Institute for Biologically Inspired Engineering, and the Howard Hughes Medical Institute (HHMI)—promises to measure cell traction forces at unprecedented resolution in space and time.
The researchers described and demonstrated this advance in the October 12 online edition of Nature Methods.
“Traditional methods that rely on watching cells pull on soft, synthetic materials could only resolve these forces at the micron or cellular scale, but the new method can do so at the nanoscale level where individual integrins adhere to the extracellular matrix, enabling researchers to obtain a much clearer picture of these forces,” said Chen, a world leader in tissue engineering and mechanobiology—the study of how physical forces and changes in cell or tissue mechanics contribute to development, physiology and disease.
With funding from the National Institutes of Health and HHMI, the research team engineered a new class of cell traction force probes consisting of single strands of DNA shaped as hairpins of different lengths and DNA sequences—each tuned to unfold when subject to a specific amount of force, and attached to a light-emitting fluorophore (a fluorescent chemical compound) and a “quencher” that effectively dims the fluorophore’s emitted light. When a cell’s integrin pulls on the extracellular matrix (ECM), the force that it exerts causes one of the DNA hairpin probes to unfold, thereby separating the quencher from the fluorophore and freeing it to emit light.
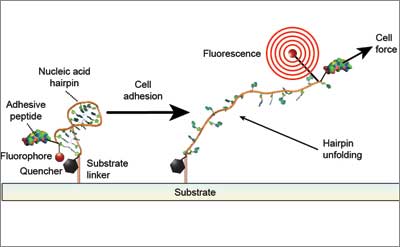
Based on the intensity of this light, a microscope measures the forces exerted by clusters of integrins at each site where a cell adheres to the ECM, and shows how these forces are distributed throughout the cell. As microscopy advances, the new method will enable measurement of cell traction forces at the single integrin level as well.
Chen conceived of the new approach with David Liu, a Harvard University professor of chemistry and chemical biology, over a long flight, and now aims to implement it to help uncover new knowledge about the mechanisms underlying cellular development.
“By engineering individual molecules as light-emitting force sensors, we hope to be able to use these as coatings to better understand how cells generate forces in a variety of settings where they have not been characterized,” he said.