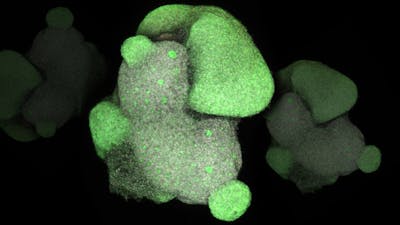
The ability to derive and manipulate pluripotent stem cells has opened up new avenues for modeling biological systems in both healthy and diseased conditions. In order to more fully recapitulate the tissue microenvironment with its cell-cell, cell-extracellular matrix, and cell-niche interactions, it is essential to transition stem-cell culturing from monolayers to 3D structures. Self-organization of a variety of cell types into 3D organoid structures that resemble human tissues such as brain, liver, intestines, kidney and optic cup has been reported, and holds great potential to elucidate how cells determine their developmental fate and how this process is affected by the genetic and environmental perturbations that cause disease.
While great advances have been made to generate more complex and multilayered 3D organoids, these large structures lack a vascular system, which is vital for delivering nutrients and oxygen, removing waste, and making these structures amenable to long-term culture. Perfusion through microfluidic systems is currently addressing this requirement, but only at the microstructure level. Furthermore, while current organoid research has been able to recapitulate some complexity, maturity, topography, and functionality of organs and organogenesis, their formation has been largely spontaneous and unguided relying mostly on extrinsic signaling from growth factors. In order to generate biomimetic tissues, all required cell types must be present at the correct proportions and with the correct cellular organization.

Researchers from the Wyss Institute and Harvard Medical School are using the human transcription factor library to generate the cell types necessary to build human cerebral organoids from induced pluripotent stem cell lines. They screen transcription factor combinations in order to determine the correct recipe to induce differentiation into a diverse repertoire of cell lineages, and endow the organoids with testable functionalities. This provides the intrinsic cellular signaling component that complements the extrinsic media conditions used in organoid work to date. Established and novel extracellular matrix and biomaterial scaffolding technologies are also being tested to aid the 3D organization of these complex structures, as well as generate an integrated vasculature network to sustain cell growth and neuronal maturation.
The aim of our work is to achieve a faithful rendering of the cerebral tissue through stem cell differentiation into vascularized organoids. We are using transcription factors to engineer cell fate and generate all the major and minor cell types at physiological ratios.
The Wyss-Harvard researchers plan to analyze the phenotypes, transcriptomes, and epigenomes of the multicellular organoids to determine their similarities to in vivo organs. The team will use the organoids for pre-clinical testing of regenerative therapies in addition to developing more sophisticated modeling of neuropsychiatric and age-related disorders in collaboration with Harvard-affiliated hospitals.