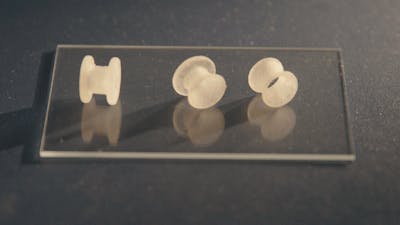
The Problem

Acute middle ear infections affect more than 700 million people each year, with children often experiencing the most recurrent and severe symptoms. By the age of three, 25-40% of children have had at least three episodes of acute middle ear infection, which is commonly accompanied by excess fluids accumulating behind the tympanic membrane (eardrum) and prolonged inflammation in the ear. Improper treatment of these infections can lead to high levels of pain, temporary or permanent hearing loss, and speech delay in children.
Recurrent ear infections and persistent fluid in the tympanic cavity are currently treated via the placement of tympanostomy tubes (commonly known as “ear tubes”) into the tympanic membrane that help equalize the pressure of and drain fluids from the middle ear. However, this approach suffers from frequent complications, including the progressive blockage of the tympanostomy tubes by cellular debris, bacterial films, ear wax, blood, and pus. This blockage prevents proper ventilation and drainage of fluids out of the middle ear, prolonging infection. Furthermore, conventional ear tubes are not designed to enable the delivery of antibiotics into the middle ear.
In the longer term, sub-optimal tympanostomy tube design can result in the tubes either falling out too early or getting stuck in the eardrum, requiring further surgeries that can permanently damage the tympanic membrane.
Our Solution
To address this challenge, a team of materials scientists at Harvard’s Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS) led by Wyss Institute Associate Faculty member Joanna Aizenberg, Ph.D., and former Technology Development Fellow Ida Pavlichenko, Ph.D. have developed a new type of customizable tympanostomy tube with rationally designed geometries created using 3D printing and injection molding techniques.
These novel tubes are coated with proprietary liquid-infused medical-grade polymers that form a frictionless, biofouling-resistant layer inside the tube, dramatically reducing the adhesion of biofluids, human cells, and common ear infection-causing bacterial strains by about 99% when compared with conventional tympanostomy tubes. Fluidic transport tests demonstrated that water, antibiotic drops, and mucus could all pass through the new tube design unhindered, and as a result the tubes themselves could be made smaller than conventional tympanostomy tubes, reducing surgical invasiveness.
Product Journey
Inspired by the highly slippery SLIPS technology created in the Aizenberg lab, Pavlichenko began exploring potential applications that could benefit from it. When her daughter developed painful, recurrent ear infections as a toddler, Pavlichenko realized that conventional tympanostomy tubes were not designed to maximize healing, and that SLIPS offered a potential solution. She iterated multiple designs and materials before landing on a flared shape, and collaborated with Dr. Aaron Remenschneider and Dr. Elliott Kozin at Massachusetts Eye and Ear to perform preclinical studies in an established chinchilla model whose results were very promising.