New method for synthesizing a biocompatible hydrogel could speed up research and development of several promising applications in tissue engineering
(BOSTON) — If you opt to wear soft contact lenses, chances are you are using hydrogels on a daily basis. Made up of polymer chains that are able to absorb water, hydrogels used in contacts are flexible and allow oxygen to pass through the lenses, keeping eyes healthy.
Hydrogels can be up to 99 percent water and as a result are similar in composition to human tissues. They can take on a variety of forms and functions beyond that of contact lenses. By tuning their shape, physical properties and chemical composition and infusing them with cells, biomedical engineers have successfully used hydrogels as three-dimensional molecular scaffolds that can be filled with cells or molecules for bodily injection or application in order to release drugs or stimulate tissue regeneration.
Alginate hydrogels — which are made up of the polysaccharide naturally occurring in brown seaweed — are just such materials. The rate at which the three-dimensional, internal molecular structure of alginates will degrade over time can be precisely tuned, which enables engineers to rationally design and control the release of drug molecules encapsulated in the gel. Wyss Core Faculty member David Mooney, Ph.D., who is also the Robert P. Pinkas Family Professor of Bioengineering at Harvards School of Engineering and Applied Sciences (SEAS), has pioneered the development of alginate hydrogels for applications such as small chemical drug delivery, tissue regeneration, stimulation of blood vessel formation, and bone and cartilage repair, among others.
But the reagents often used to make these alginate hydrogels are not chemoselective enough to be truly biocompatible. As a result, cells and molecules encapsulated in the hydrogel can become damaged during the encapsulation process or through unintended reactions with the chemical reagents in the hydrogel, making therapeutic design and delivery in the clinic very difficult.
Now, Wyss Core Faculty member Neel Joshi, Ph.D., has developed a novel, truly biocompatible alginate hydrogel in collaboration with Mooney that can be synthesized using “click chemistry”, which is a methodology for the quick and practical synthesis of substances using just a few reliable, chemoselective reagents. Joshi, who is also Associate Professor of Chemical and Biological Engineering at SEAS, leads a team at the Wyss Institute developing new synthetic biomaterials that mimic naturally-occurring materials. Joshi and Mooney’s new “click alginate” is reported in the May 1 issue of Biomaterials.
The biocompatible click alginate gels are formed using chemical crosslinking strategies that allow engineers to entrap cells or molecules inside the gel without damaging them or rendering them inactive. As such, it presents a practical platform for long-term, stable encapsulation of bioactive materials. And, it is robust enough to be used in a variety of ways, which is exciting due to the variety of therapeutic drugs that are made from chemical and protein molecules.
“It’s injectable, so it can be used to deliver cells or drugs to specific places in the body such as a location that has suffered a wound or has been invaded by a tumor,” said Joshi. “And we are already using it for lots of different things in the laboratory due to how easy it is to synthesize.”
Other types of hydrogels are much more cumbersome to synthesize, according to the study’s first author, Rajiv Desai, who is a researcher at the Wyss Institute pursuing his Ph.D. from SEAS. In contrast, the click alginate hydrogel can be created by a simple and fast combination of two simple solutions — similar to an epoxy. And once the gel is formed, the click chemistry reactions are irreversible, resulting in a chemoselective hydrogel primed for use as a therapeutic scaffold.
Furthermore, the click alginate hydrogel is easily customized and modified. “One of the many things people like to do with hydrogels is to modify them for different purposes,” said Desai. “With our new method, if you wanted to add a fluorescent dye, peptide, or protein to the new click alginate, you could do so within one minute — a truly unprecedented rate.”
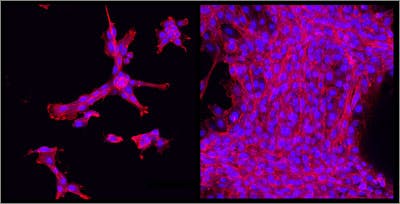
At the Wyss, the novel hydrogel is already being used to encapsulate cells in culture and to conduct experiments in a tissue-like environment. “It’s a great material for studying how cells sense the mechanical environments around them,” said Desai.
“Alginate hydrogels show promise for tissue engineering and drug delivery applications as they can be designed to dissolve away harmlessly in the body while releasing drugs, growth agents or living cells that can accelerate healing and regeneration,” said Wyss Institute Founding Director Donald Ingber, M.D., Ph.D, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital and Professor of Bioengineering at SEAS. “But what’s more, new click alginate hydrogels like the one Neel and Dave have developed can accelerate exploration and studies in the laboratory, immediately, by providing more reliable and easily-synthesized platforms for cell culture and experimentation.”
