Injectable therapy could help people avoid mastectomy
BOSTON — A novel breast-cancer therapy partially reverses the cancerous state in cultured breast tumor cells and prevents cancer development in mice, and it could one day provide a new way to treat early stages of the disease without resorting to surgery, chemotherapy or radiation, a multi-institutional team led by researchers from the Wyss Institute of Biologically Inspired Engineering at Harvard University reported January 1 in Science Translational Medicine.
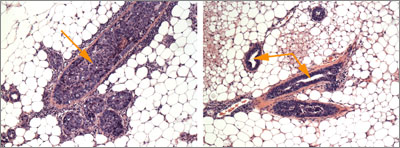
The therapy emerged from a sophisticated effort to reverse-engineer gene networks to identify genes that drive cancer. The same strategy could lead to new therapies that disable cancer-causing genes no current drugs can stop, and it can also be used to find therapies for other diseases.
“The findings open up the possibility of someday treating patients who have a genetic propensity for cancer, which could change people’s lives and alleviate great anxiety,” said Wyss Institute Founding Director Don Ingber, M.D., Ph.D. “The idea would be start giving it early on and sustain treatment throughout life to prevent cancer development or progression.” Ingber is also the Judah Folkman Professor of Vascular Biology at Boston Children’s Hospital and Harvard Medical School, and Professor of Bioengineering at the Harvard School of Engineering and Applied Sciences.
Between breast self-exams, mammograms, MRIs, and genetic tests, more women than ever are undergoing early tests that reveal precancerous breast tissue. That early diagnosis could potentially save lives; however, few of those lesions go on to become tumors, and doctors have no good way of predicting which ones will. As a result, many women undergo surgery, chemotherapy, and radiation who might never develop the disease. Moreover, some women with a high hereditary risk of breast cancer have chosen to undergo preemptive mastectomies.
A therapy that heals rather than kills cancerous tissue could potentially help all these patients, as well as men who develop the disease. But to date the only way to stop cancer cells has been to kill them. Unfortunately, the treatments that accomplish that, including surgery, chemotherapy, and radiation therapy, often damage healthy tissue, causing harsh side effects.
The Wyss Institute researchers thought they could do better by spotting new genes that drive breast cancer and developing targeted genetic therapies to block them. First they had to identify the culprit genes among the thousands that are active in a cell at any moment. Molecular biologists typically convict these culprits through guilt by association; for example, when looking for cancer-causing genes, they search for individual genes that become active as cancer develops. But because genes in cells work in complex networks, that approach has led to some false convictions, with innocent genes being fingered for crimes they did not commit.
To improve the odds of finding the real culprits, Ingber teamed up with Wyss Institute Core Faculty member Jim Collins, Ph.D., a systems biology expert who has developed a sophisticated mathematical and computational method to reverse-engineer bacterial gene networks. Collins is a Core Faculty member at the Wyss Institute for Biologically Inspired Engineering and the William F. Warren Distinguished Professor at Boston University, where he leads the Center of Synthetic Biology.
First, Hu Li, Ph.D. a former Wyss Institute postdoctoral fellow who is now an Assistant Professor of Systems Pharmacology at the Mayo Clinic, honed the computational network to work for the first time on the more complex gene networks of mice and humans. The refined method helped the scientists spot more than 100 genes that acted suspiciously just before milk-duct cells in the breast begin to overgrow. The team narrowed their list down to six genes that turn other genes on or off, and then narrowed it further to a single gene called HoxA1 that had the strongest statistical link to cancer.
The researchers wanted to know if blocking the HoxA1 gene could reverse cancer in lab-grown cells from mouse milk ducts. Amy Brock, Ph.D., a former Wyss Institute postdoctoral fellow who is now an Assistant Professor of Biomedical Engineering at the University of Texas, Austin, grew healthy mouse or human mammary-gland cells in a nutrient-rich, tissue-friendly gel. Healthy cells ensconced in the gel formed hollow spheres of cells akin to a normal milk duct. But cancerous cells, in contrast, packed together into solid, tumor-like spheres.
Brock treated cancerous cells with a short piece of RNA called a small interfering RNA (siRNA) that blocks only the HoxA1 gene. The cells reversed their march to malignancy, stopping their runaway growth and forming hollow balls as healthy cells do. What’s more, they specialized as if they were growing in healthy tissue.
The siRNA treatment also stopped breast cancer in a line of mice genetically engineered to have a gene that causes all of them to develop cancer. The Wyss team worked with Michael Goldberg, Ph.D., Assistant Professor of Microbiology and Immunobiology at Harvard Medical School and the Dana-Farber Cancer Institute, to leverage a novel method he had developed to deliver the siRNA efficiently.
They packed the siRNA into nanoparticles called lipidoids that allow for genes to be silenced for weeks inside the body. They then did something unusual: they injected these nanoparticles directly through the nipples into the milk ducts of the cancer-prone mice, using a new method that Silva Krause, Ph.D., a postdoctoral fellow on Ingber’s team, had developed. This is important because the cells that line the ducts are the ones that form breast tumors in mice as well as humans.
Weeks went by as Brock and Krause watched. The treated mice remained healthy, while untreated mice developed breast cancer. “There was no aha moment,” Brock said. “But after enough evidence builds up, you turn to each other and say this is really doing something here,” Brock said.
“We were delighted that we could reverse-engineer mammalian gene networks to identify key disease-causing genes, and we’re hopeful that our approach can help uncover new drug targets for many hard-to-treat cancers,” Collins said.
Indeed, the work marks a milestone not just in breast cancer research, but in systems biology, Ingber said. “Combining computational, engineering and biological approaches has led to a new way to identify drugs that prevent cancer development and progression.”
The work was funded by the Department of Defense Breast Cancer Innovator award provided to Don Ingber, the Howard Hughes Medical Institute, SysCODE (Systems-based Consortium for Organ Design & Engineering), the National Institutes of Health, a Susan G. Komen Foundation postdoctoral fellowship to Silva Krause, and the Wyss Institute. In addition to Brock, Krause, Li, Goldberg, Collins and Ingber, the authors included Marek Kowalski, a former research assistant at the Wyss Institute.