- Monitors a patient’s blood in real-time to predict blood clots.
- Can be used with blood samples or in-line blood flow devices.
- Machine learning algorithms predict patient risk based on hundreds of samples.
- Applicable to blood clots in a variety of contexts including surgery, organ injury, diabetes, cardiovascular diseases, autoimmune diseases, and more.
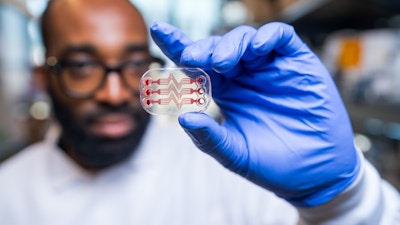
Blood Clot Dx
Rapid prediction of blood clots in patients before, during, and after surgery
Want to support this technology?
The team is currently seeking funding to clinically validate their technology for mesothelioma patients.
Bioinspired Therapeutics, Wyss Diagnostics Accelerator (DxA)
Want to support this technology?
The team is currently seeking funding to clinically validate their technology for mesothelioma patients.
The Problem
Blood clots can arise anywhere in the body, blocking blood flow and causing pain and other symptoms. The most serious types of clots, called deep vein thrombosis (DVT), typically form in the veins in the legs, and can break off and become lodged in a lung. This can cause a pulmonary embolism (PE), which is when blood flow to the lungs is prevented and can be fatal. The symptoms of PE resemble those of a heart attack, which can delay proper treatment. It’s estimated that 10-30% of people who are diagnosed with PE die within a month.
Identifying patients who are at risk for DVT before it develops into PE would be the most effective preventive treatment, but there is currently no good test for DVT before it occurs. Existing diagnostic tools suffer from low accuracy and long turnaround times, so many doctors simply don’t use them. As a result, many patients don’t know they have DVT until it develops into much more deadly PE.
Our Solution

Wyss researchers have created a microfluidic chip device that can non-invasively monitor a patient’s blood for evidence of clotting. As blood flows through a life-like network of hollow channels within the device, it is subjected to the same shear stresses and force gradients it experiences inside the patient’s blood vessels. Using automated pressure sensors and a proprietary algorithm, data acquired from the device is analyzed in real-time, precisely predicting the time at which clots will form and how quickly they will obstruct blood flow. This design enables doctors to continuously assess patients’ blood clotting status, in stark contrast to the current standard of once or twice daily testing procedures.
This device is being used to initially develop a diagnostic for patients who suffer from malignant pleural mesothelioma (MPM), an aggressive lung cancer that often requires surgery to treat. At least 33% of patients who get surgery for MPM develop DVT, making it the top cause of death in this patient population.
Wyss Effect
In 2022, as the Wyss’ Diagnostics Accelerator (DxA) program got underway, Rushdy Ahmad, Ph.D. reached out to clinicians at Brigham and Women’s Hospital asking how the Wyss’ scientists and engineers could help solve their biggest problems in practicing medicine. Among the more than 50 clinicians who responded, Raphael Bueno, M.D., Chief of the Division of Thoracic Surgery, reported that existing diagnostic tests for DVT are not very accurate and can take 24 hours or more to produce results – He dreamed of a real-time triage test that would identify MPM patients at a high risk of clotting so he could proactively put them on an anticoagulant drug after surgery to reduce their risk of DVT and death.
Ahmad put out a call to the Wyss community to identify existing Wyss technologies that could help solve Bueno’s problem. Staff Scientist Abidemi Junaid, Ph.D. realized that he had the perfect solution sitting on the shelf. Researchers in the lab of Wyss Founding Director Don Ingber created a device that could non-invasively monitor the blood for signs of clotting years prior, but the inventors had since left the lab and it had not been further developed with a specific patient focus in mind. Junaid revisited this past work and began advancing the proejct with support from a philanthropic gift through the Wyss DxA.
To test the concept, the team to start analyzing six patient samples from the BWH with the hemostasis monitor. The results were so promising that in 2024, Junaid and Adama Sesay, Ph.D., Wyss ATT member, were accepted as part of that year’s Validation Project class to further develop their technology into a clinic-ready diagnostic.
The team is currently integrating fluorescent imaging into the monitor to directly see blood clots forming in real-time, as well as a machine learning algorithm that will be able to analyze clotting data from hundreds of patients and improve the test’s predictive power.
While Junaid and Sesay have been perfecting the device through the Validation Project program, Ahmad has been leading a parallel effort through the Wyss DxA to analyze the blood samples from Bueno’s patients to identify new biomarkers that can be used to better predict DVT. These biomarkers could form the basis of a companion diagnostic test to be used alongside the device to provide even faster and more accurate answers to clinicians.
By combining our fabricated microfluidic device that mimics blood flow dynamics of small arterioles with our novel data analysis software, we can monitor blood flow in real time and predict whether deadly blood clots will develop, helping to save patients’ lives.
Impact
The team is continuing to develop and de-risk their DVT diagnostic device for use in MPM patients, with the goal of starting a clinical validation trial using a larger group of patient samples. They are currently seeking funding to support this effort and to explore their device’s potential to predict blood clots in patients who suffer from other conditions, including injuries, heart failure, diabetes, and inflammatory disorders.
This is a classic example of unmet clinical needs driving innovation, which is a central tenet of the Wyss’ Diagnostic Accelerator program. By talking directly to doctors and learning what they need, we ensure that we are solving real problems that impact patient’s lives in a very deliberate way.
Want to support this technology?
The team is currently seeking funding to clinically validate their technology for mesothelioma patients.