The Problem
There is a severe shortage of human organs for people who need transplants due to injury or disease: more than 103,000 people are on the waiting list for organs in the US, and it’s estimated that 17 people die waiting for an organ transplant every day. Growing full organs from living human cells has long been touted as a solution to this problem, but progress toward that goal has been hampered by the persistent challenge of delivering sufficient oxygen and nutrients to living tissues grown in the lab. As tissues get thicker, the cells in their interior are no longer in direct contact with the growth medium in the lab, and start to die. Solving this problem could dramatically speed up the development of implantable human tissues that can save lives.
Our Solutions
Multidisciplinary research at the Wyss Institute led by Core Faculty members Jennifer Lewis and Chris Chen has led to the development of multiple novel methods of 3D-printing living biological tissues with built-in vascular channels that ensure the delivery of critical nutrients to cells throughout a tissue. These tissues can be nearly ten-fold thicker than previously engineered tissues and remain functional for upwards of six weeks. We are pursuing several avenues of research in this area:
Multimaterial 3D-printed tissues-on-chips
One method developed in the Lewis lab co-prints multiple cell types into an organ chip structure. Within a customizable silicone mold, a grid of vascular channels containing living endothelial cells in silicone ink is printed first, into which a self-supporting ink containing living mesenchymal stem cells (MSCs) is layered. After printing, a liquid composed of fibroblasts and extracellular matrix is used to fill the gaps, adding a connective tissue component that further stabilizes the entire structure.
The resulting soft tissue structure is full of blood vessels and can be immediately perfused with nutrients via a single inlet and outlet on opposite ends of the chip to ensure survival and maturation of the cells throughout the tissue. The shape of the silicone chip and the composition of the cell “inks” can be modified to create various vascularized 3D tissues-on-chips for regenerative medicine and drug testing. This effort has resulted in the first entirely 3D-printed organ on a chip – a heart on a chip – with integrated soft strain sensors.

Dense tissues with 3D-printed vasculature
Living human tissues are incredibly dense structures, and lab-grown organs need to achieve a similar level of cell density. The Lewis lab has created another method, called SWIFT (sacrificial writing into functional tissue), in which hundreds of thousands of stem-cell-derived aggregates are concentrated into a dense, living matrix of organ building blocks (OBBs) that contains about 200 million cells per milliliter. Next, a gel-based “ink” is used to 3D print an interconnected vascular network within the matrix of cells, which is then heated to remove the gel. The result is a dense, living tissue with an embedded vasculature that can support cell growth and function throughout the tissue. When this method was used with heart cells, they started to spontaneously beat together, like a living heart. A further development of this method, co-SWIFT, improved on the original design by inventing a specialized nozzle to print a layer of endothelial cells along with the ink to replicate the natural barrier around the vasculature found in human organs..
ReConstruct: patient-derived breast tissue implants
Breast cancer survivors are faced with two bad choices when they opt for reconstruction after mastectomy: artificial implants that will inevitably fail at some point and require replacement, or a tissue flap taken from elsewhere in their bodies that can cause surgical complications. The ReConstruct project aims to give these valiant fighters a better option by isolating some of a patient’s own cells, growing them into a tissue flap, and implanting them via a novel suturable cuff that enables immediate blood flow into the new implant. Learn more about ReConstruct.
RAPID-Vasc: Rapid assembly and perfusion for implant and device vascularization
This 2024-2025 Validation Project, led by Chris Chen and Don Ingber, is developing a new approach to rapidly assemble networks of hollow channels within cell-laden hydrogels, generating high-cell-density engineered vasculature for both in vitro and in vivo applications. To learn more about RAPID-Vasc, contact Gretchen Fougere.

1/6 A method created at the Wyss called SWIFT enables the 3D-printing of hollow channels into dense matrices of living cells that can serve as a vascular network to deliver oxygen and nutrients throughout tissues. Credit: Wyss Institute at Harvard University 
2/6 A new technique that builds on SWIFT, called co-SWIFT, creates branched vascular channels to more accurately replicate the structure of naturally occurring blood vessels. Credit: Wyss Institute at Harvard University 
3/6 JThis image shows a kidney organoid (blue) embedded with vascular channels (red). Credit: Wyss Institute at Harvard University 
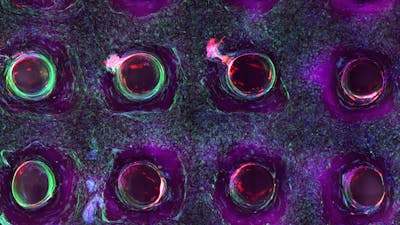
4/6 Cross section of long-term perfusion of HUVEC-lined (red) vascular network supporting HNDFladen (green) matrix. 
5/6 Photograph cross section of printed tissue construct housed within a perfusion chamber. 
6/6 Photograph of a printed tissue construct housed within a perfusion chamber.