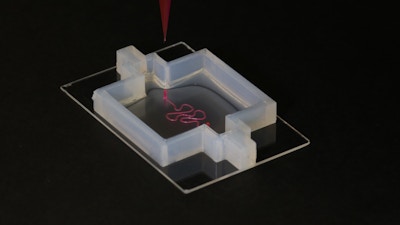
Under its ‘3D Organ Engineering’ initiative, the Wyss Institute brings together renowned faculty, towards bioengineering transplantable tissues and organs
In the U.S. alone, more than 118,000 people are in need of a lifesaving organ transplant and every ten minutes, someone new is added to the national transplant waiting list. In stark contrast, only about 9,500 donors were available between January and July 2017. Devastating numbers like those have prompted a quest to create organs in the laboratory that could be used as suitable replacements. Researchers have made progress in some of the disciplines that together may enable the multidisciplinary process of creating functional organs outside of the human body. But while human tissues have been engineered on smaller scales with first examples in clinical trials, building larger organs with multiple levels of structural organization and complex functionalities remains a grand challenge.
A multidisciplinary team of researchers and clinicians assembled at the Wyss Institute is developing novel approaches towards bioengineering transplantable tissues and organs by integrating and further expanding foundational innovations from their laboratories in areas such as tissue engineering, 3D biofabrication, biomaterials design, and stem cell differentiation. This team is led by Wyss Institute faculty members Jennifer Lewis, Sc.D., and Christopher Chen, M.D., Ph.D., and includes additional faculty members like Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., Wyss Institute faculty member David Mooney, Ph.D., as well as Sangeeta Bhatia, M.D., Ph.D., Director of MIT’s Laboratory for Multiscale Regenerative Technologies, as a collaborating investigator. The team also includes clinicians like Joseph Bonventre, M.D., Ph.D. Chief of Brigham and Women’s Hospital’s Renal Unit and Director of its Bioengineering Division, and John Mayer, M.D., Professor and Senior Associate in Cardiovascular Surgery at Boston Children’s Hospital. The recently launched effort is spearheaded by Wyss Institute Technology Development Fellow David Kolesky, Ph.D.
As a first step to organ engineering, the team aims to build branched vascular networks, whose specific organization is unique to each organ. Once in place, physical and biochemical cues and the active flow of blood-like fluid through engineered vessels can induce these blood vessels to sprout smaller vessels, allowing them to evolve into mature organ vascular systems.

After having established organ-specific vascular networks, the research team will work to develop additional methods and tools that may integrate other specialized cell types like those present in the connective tissue that provide cohesion and stability to an organ, and the cell types that perform the actual organ-specific functions, like blood filtration in the kidney, metabolism in the liver, or contraction of the heart. During that process, the engineered and induced blood vessels that reach into the depths of the 3D tissue constructs act to support the more complex nascent tissue with its cell-type specific requirements for oxygen and nutrients as well as factors cells need for their differentiation and survival.
Crucial to many steps in this process will be the Wyss Institute’s exceptional 3D bioprinting capabilities and its expertise in the design of new biomaterials that in earlier studies were demonstrated to have unique potential for tissue engineering. With advanced 3D bioprinting techniques, Institute researchers have already successfully engineered perfusable 3D vascularized tissues. With additional modifications, these were able to provide a local microenvironment for supporting the growth and maturation of a variety of tissue-specific cell types like those found in bone and kidney tissue. Other research efforts at the Institute deciphered some of the biomechanical and molecular mechanisms that blood vessels use to sprout and extend their networks and that different cells depend on, or modulate vessel permeability to control flow of fluids through tissues.
In parallel, efforts at the Wyss Institute have created smart biomaterials that can be deployed to biochemically and mechanically instruct the growth and differentiation of several cell types, like those that form bone or heart tissue. Expanding on these experiences in future approaches, biomaterials will be engineered to successfully harness the growing understanding of stem cell differentiation processes toward different functional cell types, and be deployed to provide a bioactive, biocompatible and organ-specific local environment for organ engineering. Leveraging the power of 3D bioprinting technologies, engineered biomaterials, stem cell biology, and vessel bioengineering, the team aims to combine top-down engineering principles with bottom-up developmental biology to build functional, therapeutic organ replacements.